If the reviewer deems your diagnostic as safe and efficacious then you get a letter of FDA clearance. The manufacturers of drugs and such medical devices that are required to acquire FDA approval shall include the phrase FDA Approved on their products labelling as long as such a manufacturer has received the letter from the FDA confirming such an approval.
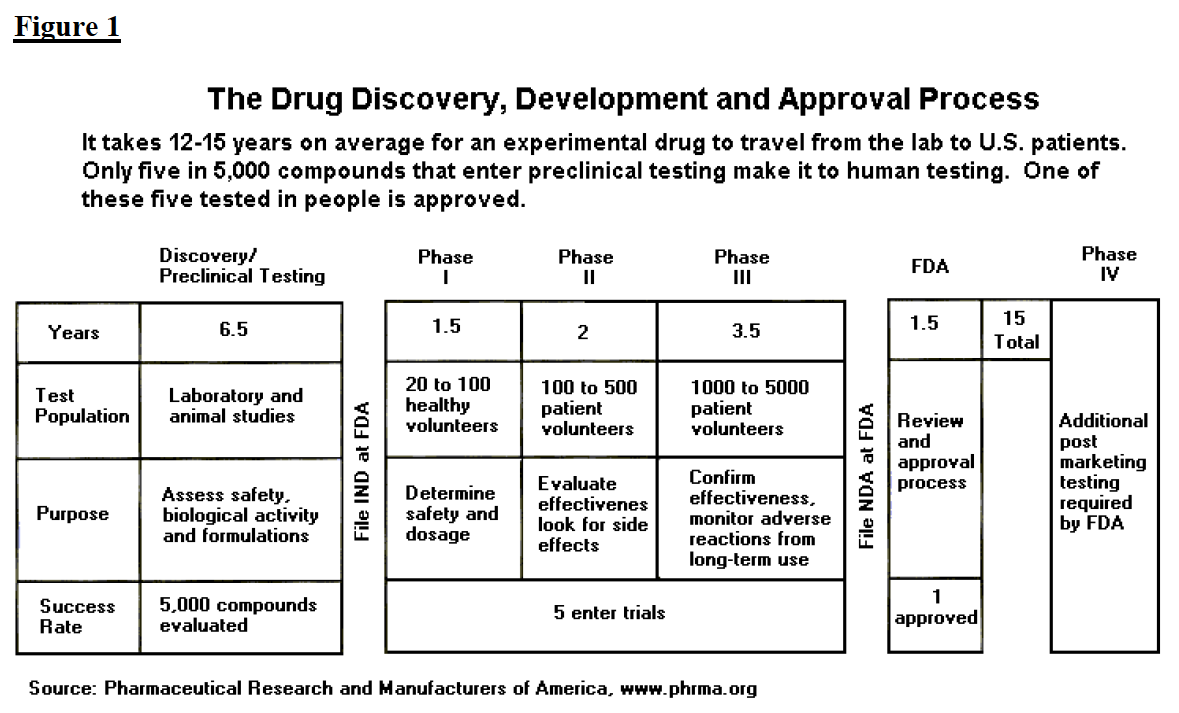 The Drug Development And Approval Process Fdareview Org
The Drug Development And Approval Process Fdareview Org
Process 1 Decide the classification of your device by examining the FDA classification database using relevant search terms or by distinguishing another device with the equivalent planned use and innovation.
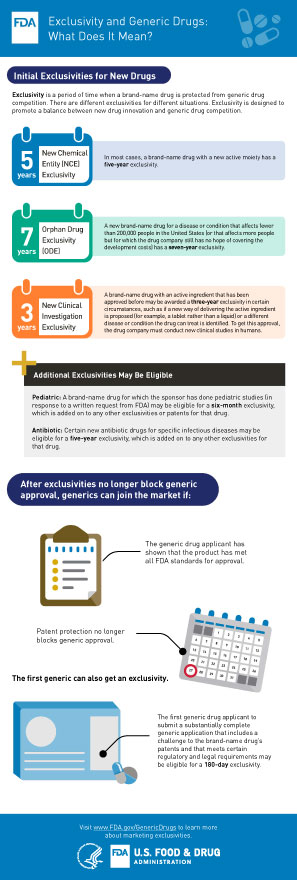
How to obtain fda approval. This prototype wont be ready for human use. However they can be marked with an FDA approved sign provided that appropriate confirmation from FDA was received. Marketing a new drug that does not conform to an OTC monograph without FDA approval is considered as marketing an.
5 days ago In general Level I or II evidence is required to obtain FDA approval of most new class III devices Table 2 20 21. A PMA is an application submitted to FDA to request approval to market. Unlike premarket notification PMA approval is to be based on a determination by FDA that the PMA contains sufficient valid.
You should know your devices classification before the development process begins. Grant special attention to the three-letter Product Code and seven-digit Regulation Number related to the predicate. 4 Pay USD to the US FDA.
To get FDA approval drug manufacturers must conduct lab animal and human clinical testing and submit their data to FDA. New Drug Application NDA For an NDA the company writes and submits an application which includes thousands of pages to the FDA for review and approval. As regards the Food and Drug Administration activities they refer mainly to documents approving a given product rather than typical certificates.
2 Fill in the Vectra FDA application form. The names and qualifications of the RDRC members and consultants 3611 c 4 a statement that the RDRC agrees to comply with the. Therefore the FDA logo cannot be placed on products and packaging even if they have been approved.
5 The agency company submits the registration application materials to the US FDA for approval. FDA review the sample of each lot of color additive manufactured and certify if it complies with requirement. For more information about how to get FDA certification or FDA approval please contact us at infofdahelpus.
How to Get FDA Approval for Medical Devices 1. Know Your Devices Classification. FDA registration for cosmetic products is not mandatory FDA does not certify or approve cosmetic products.
Would you like to learn how to sell on Amazon. To make sure that this evidence is presented correctly and that the sample group is big enough to provide significant data applicants might need the assistance of an expert statistician. Log on to FDA Industry Page FURLS at httpswwwaccessfdagovoaa with the account ID and password that you previously used to access the establishment registration that you are deactivating.
Labelling of FDA Approved Products. FDA will then review the data and may approve the drug if the agency determines that the benefits of the drug outweigh the risks for the intended use. The NDA is the official request for US approval of a drug.
FDA medical device approval process step-by-step guide. FDA batch certification is required for certain color additives. 3 Sign the contract and pay the agency fee and the US agency service is signed and effective.
The NDA includes all animal and human data plus side effects dosing and effectiveness. How to get FDA approval depends on the type of product most products do not require FDA approval to market in the United States only FDA registration or FDA clearance is required. Human drugs and therapeutic biologicals proteins and other products derived from living sources used for therapeutic purposes Drug Approval Reports by Month.
For the new drug to be approved at least 80 of subjects in the trial must show signs of improvement. How to obtain FDA approval for a product. The next step is to develop a prototype.
Its a very prescribed process where you present performance data labeling claims to an expert FDA reviewer who then may come back and ask several rounds of questions or clarifications. Complete FDA 2914 including. In order to conduct pre-market clinical trials with the device investigators must first obtain an investigational device exemption IDE summarized in Table 3 17 22.